DDL Packaging Engineers Alison Payton and Scott Levy sat down in the most recent installment of DDL’s PackReview video series to discuss some of the common questions and issues their customers have been dealing with regarding their medical device package testing and some of the recent changes with the various testing standards. The topics they covered include recent changes to ASTM F88, ASTM F1980, ASTM D4169, ISO 11607, and some of the most common questions on environmental conditioning.
ASTM F88
Many of the questions DDL has received lately regarding ASTM F88 for seal strength are about the updates with the new standard that was recently published. The biggest change with the updated standard is with tray packages. In previous versions of the ASTM F88 standard, Techniques A, B, and C offered clear testing instructions for flexible-to-flexible or semi-rigid packages (such as pouches), but it lacked guidance on conducting tests for trays made of flexible-to-rigid or semi-rigid materials. The 2023 revision has rectified this gap by providing users with guidance on conducting seal strength testing for trays.
ISO 11607
ISO 11607 is also expected to be updated again soon. The changes are likely not going to impact things much from a testing procedure standpoint but will address risk mitigation. As it was realized after the 2019 revision was published that the committee needed to beef up the “Risk Mitigation” section to further harmonize a bit closer with the MDR. This is anticipated to come out sometime this fall.
Environmental Conditioning
DDL is getting many questions on environmental conditioning, and the two primary questions we are commonly asked are: 1) Should I execute environmental conditioning? 2) How should I do it? There is no doubt that you should do it, as we have had customers tell us the FDA wants to know where their conditioning is at after submission. Performing environmental conditioning is going to better help you understand if your packaging system is robust enough to handle various temperature and humidity extremes.
The three most common standards for environmental conditioning are ASTM D4332, ASTM F2825 and ISTA 3A. Thus, the question we often get is which one should we use? As all three are consensus standards. ASTM D4332 is a standalone document, so some customers like that. The ISTA 3A conditioning profile is very similar to ASTM D4332. Thus, either one is good to use, as both are acceptable under ISO 11607 and are consensus standards by the FDA.
One of the environmental conditioning standards that has gotten people in a little bit of hot water is ASTM F2825. It is a fantastic procedure, but it is intended for next-day shipping. While it is understandable people want to get things done as quickly as possible, you must use the test procedure that best exemplifies how you intend shipping your samples. Thus, if you are shipping next day, absolutely go ahead and use ASTM F2825.
In most cases, it is best to do environmental conditioning before transportation simulation, as you want to see what happens to the samples during transportation simulation after they have gone through conditioning. While doing so may be a bit harsh, this gives you the highest assurance you are not going to run into potential problems down the line.
ASTM D4169
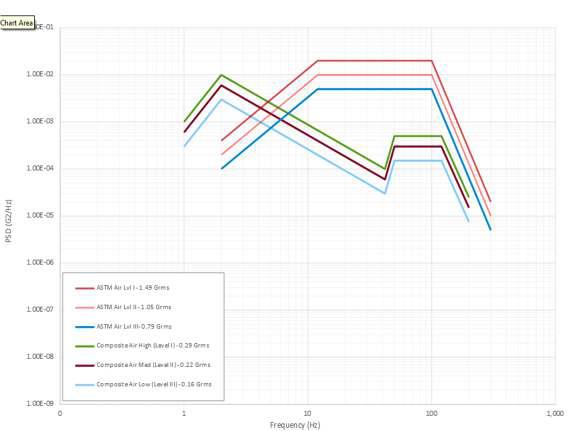
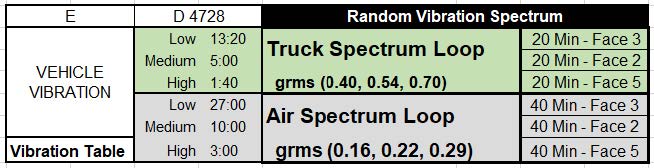
DDL has also gotten a few questions about ASTM D4169 and the differences between the 2016 revision and the 2022 revision. The newest revision modified “Schedule E” and the air spectrum vibration to better replicate actual anticipated vibratory characteristics that samples will see in air vibration. You may recall within the 2016 revision; the truck spectrum vibration was updated to provide better characterization for the truck spectrum vibration. Just like the truck spectrum vibration, the air spectrum vibration is now made up of low, medium, and high intensities and this schedule no longer uses a single assurance level. Ultimately this was done because the past vibratory characteristics were more severe than the actual events taking place. We get this question a lot: “Do I need to repeat my transit simulation to meet the new requirements of the 2022 revision?” It is DDL’s belief that the air spectrum vibration within the 2016 revision is more severe than the 2022 revision. Thus, we recommend that each organization needs to do a gap analysis to determine if they should re-execute testing or generate a rationale that what has been executed in the past can be construed as “worst case”.
ASTM F1980-21
DDL has also been getting many questions about the recently updated ASTM F1980-21 standard and the overall change with using humidity as part of the aging sequence. Traditionally, Accelerated Aging has always been about time and temperature to get your expiration date and humidity was not too much of a consideration as low humidity was primarily utilized. With the new ASTM F1980 standard, the use of specified humidity is required if the polymers that make up the package or product are considered hydrolytic. Understanding the hydrolytic aspect of your materials is probably the single biggest change we have seen with this standard in a long time. ASTM F1980 now does not just incorporate the packaging; it is incorporating the product as well. In the past, there really was not an aging standard for products. The new standard now addresses both packaging and product. This means you need to understand all of the polymers that make up your sterile barrier system and your product polymers. The easiest way to understand this is by checking with the material suppliers and further doing research to ensure you are staying in compliance with the standard. What many customers do not realize is that a justification is needed if you stay with the low humidity aging process. Essentially, for this justification, you will need to have done your research and to further show that the polymers you are utilizing are not considered hydrolytic. Ultimately, what you are concerned about if you have a hydrolytic polymer is the potential for hydrolysis. For example, if you have a hydrolytic polymer and you let it sit on the shelf for a period of time, and that polymer breaks down, what’s the result? Understanding this update is extremely important for MDMs to bring safe and effective devices to market, but it is taking MDMs a long time in many cases to chase this information.
Before starting any validation, it is imperative to understand your full packaging system and how these polymers are going to be affected. For example, if you don’t take the time to write a justification or do the research on the various polymers that make up your product and packaging system, you risk failing your validation if you simply utilize the high humidity aspect of ASTM F1980-21 and the polymers you have are not hydrolytic. Thus, it is important to spend the time and due diligence up-front to research your materials ensuring that a successful validation is executed. This is not only going to pay off with a successful validation, but will save time and eliminate rework loops, and most of all, get your product to market faster.
For more information on ASTM F88, ASTM F1980, ASTM D4169, environmental conditioning, or any other package testing issues or questions you may have, please contact us or call us at 800-229-4235.